Application Fields - DENARASE®
DNA Removal in Bioprocessing
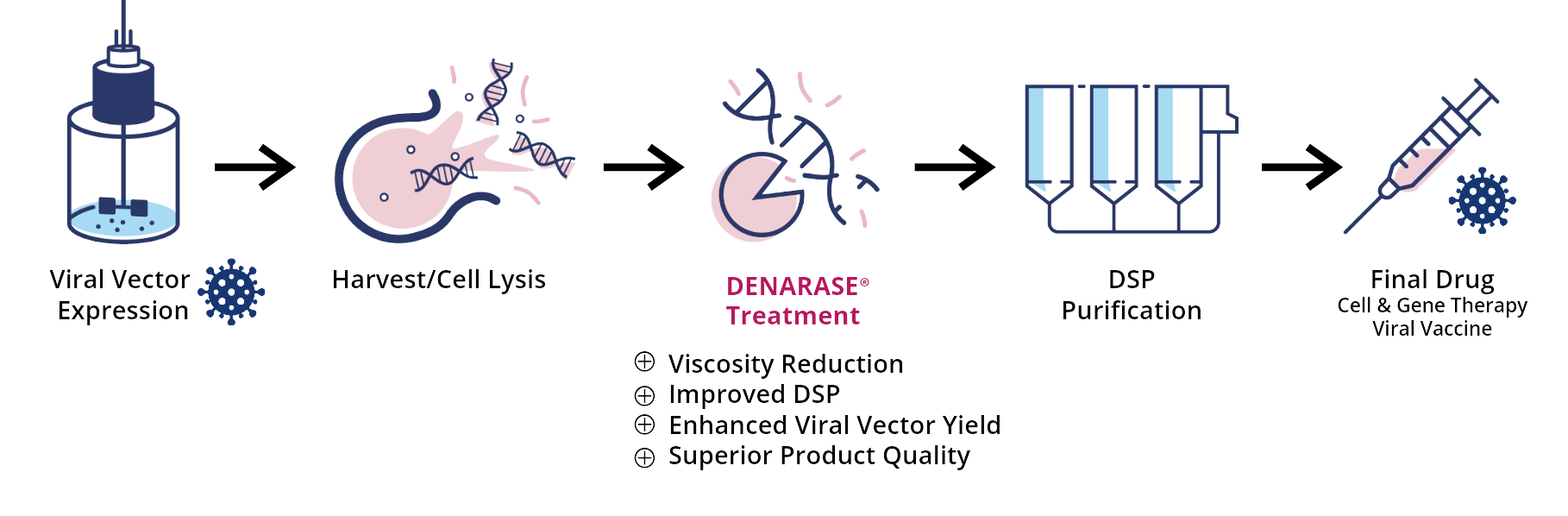
Endonucleases are commonly applied for the removal of DNA in various bioprocessing applications. These enzymes specifically digest DNA and RNA into smaller fragments of around 3-5 base pairs leaving the therapeutic molecule intact. Because of their high efficiency and specificity for nucleic acids, endonucleases from Serratia marcescens became the current industry standard for enzymatic DNA removal. Integrated into standardized workflows for viral vector manufacturing, DENARASE® enzymes facilitate downstream processing by reducing the viscosity of lysates, resulting in increased yield and purity:
Viral Vector Production for Gene Therapy
Vaccine Manufacturing
Viral vectors for gene therapy are expressed in mammalian cells such as HEK 293. After the cell culturing step, the cells are often lysed to increase the vector yield. At this stage the product is exposed to large concentrations of nucleic acids originating from the host cell as well as residual plasmids.
DNA tends to form aggregates with the product and cell debris, which makes viral vector purification challenging. Addition of DENARASE® during or immediately after the lysis step helps to rapidly reduce the amount of host-cell and plasmid DNA, without interacting with the product. This helps to improve performance of subsequent purification steps and increases the overall vector yield.
Endonucleases such as DENARASE® play an important role in vaccine manufacturing processes. In most cases they are used to reduce host-derived nucleic acids during the harvest step. Increased levels of extracellular DNA cause a higher viscosity and can build aggregates with the product, resulting in lower process efficiencies.
Addition of DENARASE® during or immediately after the lysis step rapidly reduces the free nucleic acid content and so improves the efficiency of consecutive downstream process steps. As endonucleases do not interact with proteins or viral particles, they can easily be implemented in vaccine production processes and are the best choice when process development needs to be fast.
DENARASE® is used in a range of vaccine applications using various expression systems such as E. Coli, Pichia pastoris and HEK 293 cells. Examples of processes using DENARASE® are:
-
Live-attenuated and inactivated vaccines
-
Viral Vector Vaccines (e.g, Adenovirusses)
-
Subunits for Virus Like Particles (VLP’s)
When do I need DENARASE® High Salt?
Benefits of High Salt Concentrations
-
Improved solubility: High salt concentrations (200-400 mM) enhance the solubility of viral vectors and impurities like DNA, reducing aggregate formation.
-
Enhanced DNA accessibility: Non-aggregated DNA is more accessible to endonucleases, leading to more efficient degradation.

.png)

Challenges of High Salt Levels
-
Endonuclease inhibition: Elevated salt concentrations reduce the activity of standard wild-type S. marcescens endonucleases.
-
Suboptimal alternatives: Commercial salt-active endonucleases (Salt-E) exhibit low activity and unfavorable salt and pH profiles.
DENARASE® & DENARASE® High Salt - The same, but different!
|
Characteristic |
DENARASE® |
DENARASE® High Salt |
|
Enzyme Origin |
Serratia marcescens |
Engineered from Serratia marcescens |
|
Production Host |
Bacillus sp. |
|
|
Molecular Weight |
27 kDa (per monomer) |
|
|
Temperature Optimum |
37°C |
|
|
Quantification of Residual Enzyme |
DENARASE® ELISA Kit |
|
|
Magnesium Optimum |
1-5 mM |
5-25 mM |
|
Isoelectrical Point |
pH 6.2 |
pH 7.83 |
Not quite ready to buy yet?

Get your free sample DENARASE®!
New customers from the pharmaceutical industry can request a free 25 kU sample for first lab trials.
Powerful kit for analytical support!
DENARASE® ELISA kit: For the quantitative analysis of endonucleases from Serratia marcescens.
Get in touch with our DENARASE® expert team!

Vaishnavi Devarakonda
DENARASE® Sales

Melissa Rangel
DENARASE® Sales
Need support? Contact me!

Sedef Özyürek-Heistermann
DENARASE® Sales
Need support? Contact me!